James Morrison convened another tasting at the Wine Store in Westerly. This time it was wines from Argentina. There were 4 wines being showcased including Crios Torrontes, La Posta Tinto, Tikal Patriota and Luca. Vic Zelinsky was there to transport us to Mendoza, Argentina, a place he described as being like a New World Babylon Garden.
The first wine served was the Crios Torrontes, a white wine. The nose was powerfully aromatic and the wine was crisp and delicious. I wanted to know more about the Torrontes grape so I turned to the 3 J's (I read it on some one's blog, Jancis, Julia and José) new book, Wines Grapes. I learned that there are three Torrontes grapes in Argentina. Torrontes Riojano is the most important, followed by the two lesser known Torrontes Mendocino and Torrontes Sanjuanino. Through DNA analysis, it has been shown that Torrontes Riojano and Torrontes Sanjuanino is a cross between Muscat of Alexandria and Listan Prieto and that Torrontes Mendocino is descended from Muscat of Alexandria and an unidentified variety. All three grapes belong to the Criolla group.
The second wine that was poured was the 2010 La Posta Cocina Tinto, a blend of 60% Malbec, 20% Syrah and 20% Bonarda. Vic explained that the wine was made from grapes from various vineyards and that label refers to the fact that the growers met at a tavern (La Posta) to discuss all things wine. We next tried the Tikal Patriota 2011, a blend of malbec and bonarda. I knew that malbec is the red wine of Argentina but I had no idea what bonarda was. Vic mentioned that it came from the Piedmont region of Italy. I thought that it had the deep inkiness of a Barbera (too much Barbera on the mind!) I looked up bonarda in Wines Grapes and found the following information: Bornarda Piemontese is often confused with Douce Noire, known as Bonarda in Argentina. In the U.S., Bonarda is also known as Charbono! DNA analysis showed that the origin of the Argentinian Bonarda is most likely Savoie, located in eastern France. The last wine that we tasted was a Luca made from 100% Malbec. We always enjoy going to James' wine tastings, it's a nice way to taste different wines and meet like minded people.
References:
1. J. Robinson, J. Harding and J. Vouillamoz, Wine Grapes - A complete guide to 1,368 vine varieties, including their origins and flavours.
Pages
- Welcome
- Home
- Online Resources
- Starting a Vineyard
- Viticulture
- Enology
- Grape and Wine Chemistry
- Grapevine Diseases
- Insect Pests and Predators
- Vintage Notes
- Wine Tastings
- Wines Under $20
- Champagne
- Chenin Blanc
- Meetings and Workshops
- Book Reviews
- On the Wine Trail
- Esoterica
- Vineyard Sightings
- Winery Configuration
Thursday, October 31, 2013
Monday, October 28, 2013
Frost Friday and Sowing Oats
Our first frost event happened on Friday morning. We are still sowing cover crop seeds so we headed off to the land first thing Saturday morning. We finished sowing 17 rows of oats, nitro radish and hairy vetch by 1 p.m. This was a good thing because the winds really began to kick up and by mid-afternoon, it was howling! The good news is that all of our cover crop seeds have been sown and even better, we had a rain event early Sunday morning. Hopefully the rain will aid in the germination of the seeds. We are alternating our 35 row vineyard with 2 mixtures of seeds: (1) winter oats bob, crimson clover and nitro radish and (2) winter rye, hairy vetch and nitro radish.
The following drawings and information comes from the online publication Managing Cover Crops Profitably from the Sustainable Agriculture Research and Education (SARE) site:
The other seed that is in both mixtures is called nitro radish. Nitro radish is also known as Raphanus sativus, but we Japanese also know it as daikon. We incorporated nitro radish in our cover crop mixture because it is important as a bio-driller, biofumigant, and is also used in erosion control and nematode control, and is effective in nutrient scavenging.
The following drawings and information comes from the online publication Managing Cover Crops Profitably from the Sustainable Agriculture Research and Education (SARE) site:
| Oats or Avena sativa is a cool season annual cereal that is used for suppressing weeds, preventing erosion, scavenging excess nutrients, and adding biomass. | |
| Crimson clover or Trifolium incarnatum is a winter annual or summer annual legume that is used for providing a N source, building soils, preventing erosion, and reseeding inter-row ground cover. | |
| Winter (cereal) rye or Secale cereale is a cool season annual cereal grain that is used for scavenging excess N, preventing erosion, adding organic matter, and suppressing weeds. | |
| Hairy vetch or Vicia villosa is a winter annual or summer annual legume that is used for providing a N source, suppressing weeds, conditioning the topsoil, and reducing erosion. |
Friday, October 25, 2013
Dinner and Wine Fest at Ella's in Westerly
Last night, we joined our friends for a treat at Ella's Fine Food and Drink in Westerly, Rhode Island. I think this event was the brain child of James Morrison from The Wine Store, also in Westerly, whose wine selections were the basis of the 6 course meal extravaganza created by chef-owner Jeanie Roland. James Roland (husband and owner of Ella's) and James Morrison were there to welcome the guests who packed the dining room.
The preview of the red wines that were to be served at the dinner was laid out on the table as guests filed in. The meal began with appetizers and champagne. I have to confess, I left my menu at the restaurant so I'll go from what I remember---may get more vague as I progress. The first course was a seared tuna served with Hugel Gewurztraminer, second course seared scallop with Louis Latour Puligny-Montrachet, third course, duck with Ondines Vacqueyras, fourth course braised short ribs with Clos Siguier Cahors and lastly dessert was a chocolate mousse with a 1997 Offley Port.
It was quite the evening! Great job Jeanie, James and James!
Wednesday, October 23, 2013
Turkeys vs. Tractor
Not all the time that we spend in the vineyard can be classified as work. Sometimes, we are amazed at what will appear. Yesterday, while my husband was on his tractor and I was picking rocks, he let me know that I needed to look in his direction. There they were and one was actually taunting him: "Hey, farmer, wanna race?"
It took me a while to whip out my camera to catch this amazing (to us) flock of turkeys walking from the south side of our vineyard.
That's what draws us to our vineyard---you never know what the day will bring while tending the vines!
Tuesday, October 22, 2013
Soil pH Adjustment with Lime
I'm trying (still) to understand the meaning of our soil report we recently received, specifically as it relates to the values that we obtained for our cation exchange capacity (CEC), base saturation, nutrient anions and organic matter. In today's blog, I'm going to delve a little into pH adjustment.
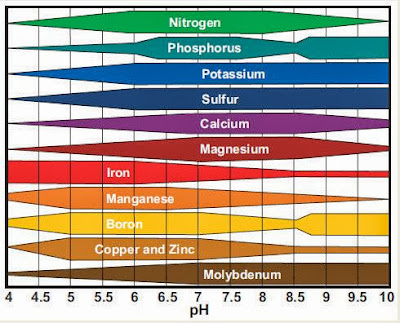
Last December, we limed our soil to bring the pH from a starting value of 5.3 to a more neutral pH (closer to 7). The proper soil pH is important to ensure the availability of certain plant nutrients as shown in the following graph:1
An article I found online called Liming of Vineyard Soils, mentions that the amount of pH change desired and the type of clay mineral present also affects the amount of lime needed to change the pH. In addition, there is a relationship between soil texture, cation exchange capacity (CEC), and buffering capacity (resistance to a change in ion concentration) that affect the amount of lime needed to change the pH.2
There are also different types of lime that can be used. But in all cases, the purpose of the liming material containing either OH- or HCO3-, is to neutralize the H+ ion in the soil solution. Pure calcium carbonate (CaCO3) is used to define the acid-neutralizing capacity of the liming material and it is expressed as a weight percentage of CaCO3. The calcium carbonate equivalent (CCE) is the standard against which other liming materials are measured, and its neutralizing value is considered to be 100%. The reaction that occurs in the soil is the following: From our soil analysis, we knew that we had enough magnesium in our soil, so our choice was to get lime that contained more calcium. We chose a pelletized calcite that contained 95.8% CCE made up of 87.5% calcium carbonate (CaCO3) and 49% calcium oxide (CaO).
References:
1. Mark Chien, Grapevine Nutrition, Penn State, College of Agricultural Sciences.
2. Thomas J. Rice, PhD, Liming of Vineyard Soils, Practical Winery & Vineyard Journal, July-August, 1999.
There are also different types of lime that can be used. But in all cases, the purpose of the liming material containing either OH- or HCO3-, is to neutralize the H+ ion in the soil solution. Pure calcium carbonate (CaCO3) is used to define the acid-neutralizing capacity of the liming material and it is expressed as a weight percentage of CaCO3. The calcium carbonate equivalent (CCE) is the standard against which other liming materials are measured, and its neutralizing value is considered to be 100%. The reaction that occurs in the soil is the following: From our soil analysis, we knew that we had enough magnesium in our soil, so our choice was to get lime that contained more calcium. We chose a pelletized calcite that contained 95.8% CCE made up of 87.5% calcium carbonate (CaCO3) and 49% calcium oxide (CaO).
References:
1. Mark Chien, Grapevine Nutrition, Penn State, College of Agricultural Sciences.
2. Thomas J. Rice, PhD, Liming of Vineyard Soils, Practical Winery & Vineyard Journal, July-August, 1999.
Saturday, October 19, 2013
Results of Our Fall Soil Tests
We took more soil samples a few weeks ago to see if the liming that we did in December 2012 had an impact on raising our soil pH which was about 5.3. We took soil samples based on how we thought our vines were performing and divided the field into octants, roughly based on our grape variety. Interestingly, the pH of our soil where our Chardonnay, clone 96 is planted on the extreme east side of our field, was at a lower pH (5.5) than the rest of the field, with the exception of one block of Chenin blanc on the south side of our field, (I don't quite understand why this is the case). We made an Excel spreadsheet of the results from the beginning of our soil sampling to see if we could see any other trends, especially for potassium, zinc, sulfur and boron, which was also incorporated in the soil at the same time that we did the liming.
Mark Chien has a great presentation called Grapevine Nutrition1 where he advises how much of the macro- and micronutrients should constitute an appropriate level or range so we added that information to the spreadsheet so that it would be easy to decipher if we had the appropriate levels: My husband, being an analytical chemist by training was way ahead of me in deciphering the soil analysis report. He determined what kind of lime we needed based on the soil report. In order to provide informed data for this blog, I pulled out our copy of Wine Grape Production Guide for Eastern North America and turned to the chapter on Nutrient Management. There I found a good explanation for cation exchange capacity (CEC), base saturation, nutrient anions and organic matter, which are additional information included in the soil analysis. It was very complicated. I'll write more about the interactions between pH, CEC, base saturation and organic matter soon.
References:
1. Mark Chien, Grapevine Nutrition, Penn State, College of Agricultural Sciences.
2. Wine Grape Production Guide for Eastern North America, Tony Wolf (Editor), published 2008.
Mark Chien has a great presentation called Grapevine Nutrition1 where he advises how much of the macro- and micronutrients should constitute an appropriate level or range so we added that information to the spreadsheet so that it would be easy to decipher if we had the appropriate levels: My husband, being an analytical chemist by training was way ahead of me in deciphering the soil analysis report. He determined what kind of lime we needed based on the soil report. In order to provide informed data for this blog, I pulled out our copy of Wine Grape Production Guide for Eastern North America and turned to the chapter on Nutrient Management. There I found a good explanation for cation exchange capacity (CEC), base saturation, nutrient anions and organic matter, which are additional information included in the soil analysis. It was very complicated. I'll write more about the interactions between pH, CEC, base saturation and organic matter soon.
References:
1. Mark Chien, Grapevine Nutrition, Penn State, College of Agricultural Sciences.
2. Wine Grape Production Guide for Eastern North America, Tony Wolf (Editor), published 2008.
Wednesday, October 16, 2013
Vineyard Work in the Fall
I was talking with the morning group that goes to the Y to exercise and mentioned how the vineyard did this year. Due to a certain amount of naivety, we were caught off guard when summer came and July was hot and humid and promoted weed and mildew growth but not vine growth. We quickly followed up with a soft spray regime recommended by Fritz Westover, our vineyard consultant, to beat down the mildews. That's when I learned that one gallon of water weighs 8.35 pounds. My husband bought us a portable sprayer that straps on to our backs and can carry approximately 4 gallons of liquid and is relatively on the light side at 11.5 pounds. So, a completely full back sprayer would weigh approximately 45 pounds! I was going to write a blog about it and call it "He Ain't Heavy, He's My Birchmeier" or "The Unbelievable Heaviness of Water" but I held back. When I mentioned this to the morning Y-goers, one person said, "making wine is not all that romantic is it?" I couldn't think of a response at that moment, but upon some reflection, I'd have to say although there may not be romance in the vineyard, there is certainly a lot of passion.
This fall finds us taking soil samples and determining what we need for soil remediation. We're still working the vineyard alleys to remove the crab grass that grew in order to spread our cover crop seeds. Fortunately for us, Nature has bestowed this fall with a glorious Indian summer.
This fall finds us taking soil samples and determining what we need for soil remediation. We're still working the vineyard alleys to remove the crab grass that grew in order to spread our cover crop seeds. Fortunately for us, Nature has bestowed this fall with a glorious Indian summer.
Monday, October 14, 2013
2009 G.D. Vajra Barbera d'Alba
We followed up the 2009 Castello di Neive Barbera d'Alba with the 2009 G.D. Vajra Barbera d'Alba. The color was intensely purple with a generous amount of fruit and well balanced acids which went seamlessly with the Mediterranean style fish (black olives, roasted red peppers, and artichokes) that my husband made. He claims that it was his own creation. It (wine and dinner) was delicious! I found some information on G.D. Vajra at Martin-Scott Wines. G. D. Vajra was founded in 1972 by husband and wife Aldo and Milena Vaira and named in honor of Aldo's father, Giuseppe Domenico.
What resonanted with me was the following, "The wines are made from 100% estate fruit that is triple sorted by hand." The hand-picked fruit was fermented and macerated in temperature controlled stainless steel tanks for 20 days with manual punch down and aged for 20-24 months in Slavonian oak casks. No wonder it was so lusciously delicious.
Saturday, October 12, 2013
2009 Castello di Neive Barbera d'Alba
We're still trying to decide what kind of red grape varieties to plant, so we are at "it" again, "it" being doing research and research in this case consists of ordering a case of various Italian Barberas and tasting through them. I know, it's difficult research but someone has to do it. In 2012, we went to the Sierra Foothills and spoke with Ann Kraemer and Dick Cooper about growing Barbera. The lesson we learned there is that Barbera is a grape variety that has a surfeit of acid and getting the proper balance between sugar and acid is important if we want to make quality wine.
We followed up the trip to the Sierra Foothills with a tasting of various Italian Barberas, but we remained unconvinced. This new tasting is changing our minds. We began with the 2009 Castello di Neive Barbera d'Alba from the Santo Stefano vineyard. When we first opened the bottle, I thought the wine was a little rustic, not a refined wine like say, a Nebbiolo. Two days later, we had this wine with garlic infused olive oil on semolina batard with fresh tomatoes and mozzarella and Italian blend cheese which my husband broiled. This combination worked. The wine was a perfect pairing with the simple food. The technical sheet for the 2009 Castello di Neive Barbera d'Alba mentions that the wine is made from grapes from the 3.7 acre Santo Stefano vineyard and produced 13,000 bottles.
We followed up the trip to the Sierra Foothills with a tasting of various Italian Barberas, but we remained unconvinced. This new tasting is changing our minds. We began with the 2009 Castello di Neive Barbera d'Alba from the Santo Stefano vineyard. When we first opened the bottle, I thought the wine was a little rustic, not a refined wine like say, a Nebbiolo. Two days later, we had this wine with garlic infused olive oil on semolina batard with fresh tomatoes and mozzarella and Italian blend cheese which my husband broiled. This combination worked. The wine was a perfect pairing with the simple food. The technical sheet for the 2009 Castello di Neive Barbera d'Alba mentions that the wine is made from grapes from the 3.7 acre Santo Stefano vineyard and produced 13,000 bottles.
Thursday, October 10, 2013
Strategies to Manage Dissolved Oxygen
My previous two blogs, How to Prevent Premox in White Wines and Chemical Marker Sotolon Found in Premox Wines gave oxygen a bad name, but at certain points in the transition of must to wine, oxygen is necessary. I found a helpful article in Wines & Vines written by T.E. Steiner called Strategies to Manage Dissolved Oxygen.
Concentrations of oxygen in the initial must are in the range of 4-6 mg/L. This amount of oxygen is essential in the early stages of fermentation when the yeast is in the growth phase. In fact, yeast consume the majority of oxygen which, happily for winemakers, results in the fermentation of sugar to ethanol. The problem comes when there is no sulfur dioxide (SO2) at this point because the following potential negative effects can also occur:
References:
1. Jackson, Ronald S., Wine Science: Principles and Applications, Third Edition, Elsevier, Academic Press, 2008, pg. 298.
2. T.E. Steiner, Strategies to Manage Dissolved Oxygen, Wines & Vines, August, 2013.
Concentrations of oxygen in the initial must are in the range of 4-6 mg/L. This amount of oxygen is essential in the early stages of fermentation when the yeast is in the growth phase. In fact, yeast consume the majority of oxygen which, happily for winemakers, results in the fermentation of sugar to ethanol. The problem comes when there is no sulfur dioxide (SO2) at this point because the following potential negative effects can also occur:
- Enzymatic oxidation
- Polyphenol oxidase (PPO) oxidizes certain phenolic molecules in juice and must, but not wine, to produce quinones
- Laccase from Botrytis cinera or bunch rot, is an enzyme that has a wide range of oxidative substrates and is more resistant to SO2
- Chemical oxidation
- The reaction of oxygen with certain phenols form quinones and hydrogen peroxide (H2O2), a stronger oxidizing agent that PPO.
- H2O2 also converts ethanol to acetaldehyde, resulting in a sherry-like aroma
- Microbial oxidation
- Acetic acid bacteria (AAB), Candida and Brettanomyces are dependent upon oxygen. In the presence of oxygen, AAB can produce acetic acid from ethanol as well as acetaldehyde and ethyl acetate.
- Certain "wild" yeasts belonging to the group Kloeckera and Hanseniaspora can be abundant in the must during the beginning states of fermentation and can produce high levels of acetic acid and ethyl acetate.2
References:
1. Jackson, Ronald S., Wine Science: Principles and Applications, Third Edition, Elsevier, Academic Press, 2008, pg. 298.
2. T.E. Steiner, Strategies to Manage Dissolved Oxygen, Wines & Vines, August, 2013.
Tuesday, October 8, 2013
Turkeys in the Vineyard
Yesterday, we drove up to our land at mid-day and saw our vineyard turkeys at the entryway. We have seen these birds grow from little poults and now they are just ready for eating! (Just joking, although wild turkey is delicious!) They regularly come into our vineyard and do bug patrol for us. We've seen them mainly at dusk on the north portion of the vineyard. It is okay for now since we don't have any grapes.
One day, they formed a line and were meticulously eating what they found in the soil beginning from the northeast corner and were working their way down to the southeast corner. They were spooked when my husband got on his tractor and started cultivating the west headlands.
One day, they formed a line and were meticulously eating what they found in the soil beginning from the northeast corner and were working their way down to the southeast corner. They were spooked when my husband got on his tractor and started cultivating the west headlands.
Monday, October 7, 2013
Chemical Marker Sotolon Found in Premox Wines
Following up on the previous blog about How to Prevent Premox in Wines, there appeared another article published by Professor Denis Dubourdieu and Dr. Valérie Lavigne, which can be found in it's entirety at The New Bordeaux entitled The premature oxidative ageing of wine. The article is very accessible and entertaining in parts but also addresses the profound question of premature oxidative (premox) aging in white wines.
Beginning in 2000, Professor Denis Dubourdieu and Dr. Valérie Lavigne, using gas chromatography, conducted research into the aroma compounds that constitute a wine suffering from premox. Three compounds methional, phenylacetaldehyde and o-aminoacetophenone had already been identified in prematurely aged dry white wines but not all premoxed wines contain these compounds. One compound, sotolon was identified by Dubourdieu and Lavigne to contibute to the aroma of all premoxed wines. Sotolon is a volatile heterocyclic compound with an intense curry aroma with a detection threshold in dry white wines on the order of 7 μg/L (7 ppb). The sotolon molecule has an asymmetrical or chiral carbon atom as shown in the depiction below. The chiral carbon in sotolon means that it has two enantiomeric forms, R and S. The R and S forms of sotolon confer different attributes to the molecule. (S)-sotolon is much more readily detected and the odor is reminiscent of curry and walnuts, while(R)-sotolon smells only of rancid walnuts.
The authors go on to write that since sotolon is formed by a reaction between α-ketobutyric acid, present in all wines, and acetaldehyde, whose concentration is increased by oxidation, it is absolutely essential for winemakers to avoid acetaldehyde production at all stages of white wine production.
References:
1. Denis Dubourdieu and Valérie Lavigne, The premature oxidative ageing of wine, The New Bordeaux.
2. Chemical structure and IUPAC nomenclature of sotolon retrieved from Chemicalize.org. All structures were drawn by the freely available drawing program from ACD Labs called ACD/ChemSketch Freeware.
Beginning in 2000, Professor Denis Dubourdieu and Dr. Valérie Lavigne, using gas chromatography, conducted research into the aroma compounds that constitute a wine suffering from premox. Three compounds methional, phenylacetaldehyde and o-aminoacetophenone had already been identified in prematurely aged dry white wines but not all premoxed wines contain these compounds. One compound, sotolon was identified by Dubourdieu and Lavigne to contibute to the aroma of all premoxed wines. Sotolon is a volatile heterocyclic compound with an intense curry aroma with a detection threshold in dry white wines on the order of 7 μg/L (7 ppb). The sotolon molecule has an asymmetrical or chiral carbon atom as shown in the depiction below. The chiral carbon in sotolon means that it has two enantiomeric forms, R and S. The R and S forms of sotolon confer different attributes to the molecule. (S)-sotolon is much more readily detected and the odor is reminiscent of curry and walnuts, while(R)-sotolon smells only of rancid walnuts.
The authors go on to write that since sotolon is formed by a reaction between α-ketobutyric acid, present in all wines, and acetaldehyde, whose concentration is increased by oxidation, it is absolutely essential for winemakers to avoid acetaldehyde production at all stages of white wine production.
References:
1. Denis Dubourdieu and Valérie Lavigne, The premature oxidative ageing of wine, The New Bordeaux.
2. Chemical structure and IUPAC nomenclature of sotolon retrieved from Chemicalize.org. All structures were drawn by the freely available drawing program from ACD Labs called ACD/ChemSketch Freeware.
Saturday, October 5, 2013
How to Prevent Premox in White Wines
My husband recently sent me this link: FERMENTATION KEY TO PREMOX BATTLE, an article that appeared in the drinks business on September 26, 2013.
Since we will be making Chardonnay, I was interested in what Professor Denis Dubourdieu and Dr. Valérie Lavigne had to say about how to prevent premature oxidation. Based on their research, Dr. Lavigne listed seven key moments in the winemaking process when grapes can become especially susceptible to oxidation:
Since we will be making Chardonnay, I was interested in what Professor Denis Dubourdieu and Dr. Valérie Lavigne had to say about how to prevent premature oxidation. Based on their research, Dr. Lavigne listed seven key moments in the winemaking process when grapes can become especially susceptible to oxidation:
- In the vineyard, the lack of nitrogen diminishes plant vigour and diminishes the levels of glutathione in grapes, a compound which protects against oxidation
- During pressing, limit the extraction of phenolic compounds since excessive levels will lower the amount of glutathione in the juice
- During fermentation, the must should not be too clean or the fermentation will be too slow or incomplete leading to the loss of reductive qualities
- During fermentation, ensure that only a short period elapses between alcoholic and malolactic fermentation, a critical time when the wine is not protected by carbon dioxide or sulfur dioxide
- During barrel aging, battonage helps to create a more reductive atmosphere
- During barrel aging, it is important to protect the wine with inert gas and sulphur dioxide
- During bottling, limit the wine’s exposure to oxygen
Friday, October 4, 2013
Harvest in Southeastern New England
We went to help our friend, Dave, who is the vineyard manager at Saltwater Farm Vineyard pick his Chardonnay grapes. We arrived around 8 a.m. and our friend Barry was already there. We met the owner, Michael Connery and James, Dave's assistant gave us our harvest shears and directed us to the rows that we would be working on. The yellow picking lugs were already laid out and there were people already working. The morning was cool, perfect for picking grapes. More people arrived as the morning wore on and I overheard James say that there were almost 30 people helping to harvest. Everyone was enjoying picking grapes. I heard one person say that she loved harvest so much that she might have to move to California.
We may have picked a total of 9 lugs in 2 hours between the 3 of us. It sounds really slow going but we were following James' instructions to knock out the rot on the grapes when we saw it. This kind of field work is essential in order to bring in the best possible grapes for pressing. As we left, we saw Dave transporting the stainless steel fermenter to Stonington Vineyards who would be pressing the grapes for him. The grapes in the yellow lugs were in a separate truck not far behind. It's a fun time in the vineyard. We'll be there again this morning.
We may have picked a total of 9 lugs in 2 hours between the 3 of us. It sounds really slow going but we were following James' instructions to knock out the rot on the grapes when we saw it. This kind of field work is essential in order to bring in the best possible grapes for pressing. As we left, we saw Dave transporting the stainless steel fermenter to Stonington Vineyards who would be pressing the grapes for him. The grapes in the yellow lugs were in a separate truck not far behind. It's a fun time in the vineyard. We'll be there again this morning.
Tuesday, October 1, 2013
Insect Pests of Grapevines
Fall is rapidly arriving, actually some might say that it arrived soon after Labor Day, when like a light switch, the evening temperatures dropped to 50 degrees and the daytime high was hovering around the high 70s. But I preferred to think that it was still summer until the fall equinox, which occurred on September 22, 2013 at 4:44 P.M EDT.
Today is October 1st and it's a top ten fall day. We were out working in the vineyard. We are seeing less and less insects so I thought I would review some of the insect pests that we saw this first year in our vineyard. Some of the pests like the Japanese beetles were really destructive, actually they were all pretty destructive, but some like the Pandorus sphinx moth were quite beautiful. I have to admit that I took one home and tried to raise until it pupated, sacrificing some Vitis vinifera leaves in the process. It grew huge and morphed into it's last instar where the little horn disappeared then I read that the caterpillar likes to dig a hole in the soil to pupate. I knew my husband wasn't going to allow me to bring in soil in a jar so I let the caterpillar go. It was only too happy to find itself on solid ground and made fast work of putting distance between me and freedom.
Today is October 1st and it's a top ten fall day. We were out working in the vineyard. We are seeing less and less insects so I thought I would review some of the insect pests that we saw this first year in our vineyard. Some of the pests like the Japanese beetles were really destructive, actually they were all pretty destructive, but some like the Pandorus sphinx moth were quite beautiful. I have to admit that I took one home and tried to raise until it pupated, sacrificing some Vitis vinifera leaves in the process. It grew huge and morphed into it's last instar where the little horn disappeared then I read that the caterpillar likes to dig a hole in the soil to pupate. I knew my husband wasn't going to allow me to bring in soil in a jar so I let the caterpillar go. It was only too happy to find itself on solid ground and made fast work of putting distance between me and freedom.
| |
|
| |
|
| |
Subscribe to:
Posts (Atom)