I'm trying (still) to understand the meaning of our soil report we recently received, specifically as it relates to the values that we obtained for our cation exchange capacity (CEC), base saturation, nutrient anions and organic matter. In today's blog, I'm going to delve a little into pH adjustment.
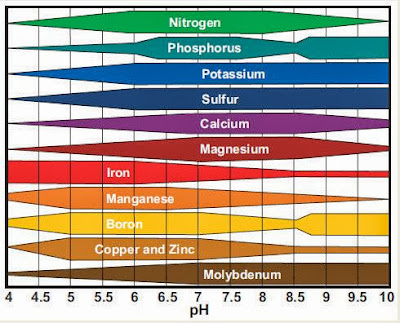
Last December, we limed our soil to bring the pH from a starting value of 5.3 to a more neutral pH (closer to 7). The proper soil pH is important to ensure the availability of certain plant nutrients as shown in the following graph:1
An article I found online called Liming of Vineyard Soils, mentions that the amount of pH change desired and the type of clay mineral present also affects the amount of lime needed to change the pH. In addition, there is a relationship between soil texture, cation exchange capacity (CEC), and buffering capacity (resistance to a change in ion concentration) that affect the amount of lime needed to change the pH.2
There are also different types of lime that can be used. But in all cases, the purpose of the liming material containing either OH- or HCO3-, is to neutralize the H+ ion in the soil solution. Pure calcium carbonate (CaCO3) is used to define the acid-neutralizing capacity of the liming material and it is expressed as a weight percentage of CaCO3. The calcium carbonate equivalent (CCE) is the standard against which other liming materials are measured, and its neutralizing value is considered to be 100%. The reaction that occurs in the soil is the following:
From our soil analysis, we knew that we had enough magnesium in our soil, so our choice was to get lime that contained more calcium. We chose a pelletized calcite that contained 95.8% CCE made up of 87.5% calcium carbonate (CaCO3) and 49% calcium oxide (CaO).
References:
1. Mark Chien, Grapevine Nutrition, Penn State, College of Agricultural Sciences.
2. Thomas J. Rice, PhD, Liming of Vineyard Soils, Practical Winery & Vineyard Journal, July-August, 1999.

No comments:
Post a Comment