- the grapes
- the yeast
- either fermentation or storage in oak barrels
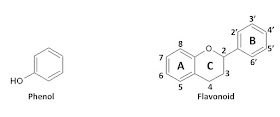
In my previous blog on the nonflavonoid phenols, the hydroxycinnamic acids and their corresponding cinnamates had in common, the "phenol" chemical moiety shown below on the left. The flavonoids also called diphenylpropanoids will have in common the chemical moiety shown below on the right.1 The flavonoid structure can be modified to yield flavan-3-ols, flavonols, and anthocyanins. These compounds all come from the grape. In my next blog, I'll elaborate on the compounds found in grapes that are members of the flavan-3-ol family. Helpful hint: You can see a larger version of the structure by clicking on the image.
References:
1. Douglas Adams, VI257, Lesson 8, pg. 21.
All structures were drawn by the freely available drawing program from ACD Labs called ACD/ChemSketch Freeware.

3'-Geranyl-3-prenyl-2',4',5,7-tetrahydroxyflavone is a natural flavonoid found in the root bark of Morus alba. 3\'-Geranyl-3-prenyl-2\',4\',5,7-tetrahydroxyflavone
ReplyDeleteGreat tips regrading flavonoids. You provided the best information which helps us a lot. Thanks for sharing the wonderful information.
ReplyDeleteThis comment has been removed by a blog administrator.
ReplyDeleteThank you for your comment. I appreciate that you find this blog helpful.
ReplyDelete